
Code: 04579244
Risk-Characterization Framework for Decision-Making at the Food and Drug Administration
by Committee on Ranking FDA Product Categories Based on Health Consequences, Phase II, Board on Environmental Studies and Toxicology, Institute of Medici
With the responsibility to ensure the safety of food, drugs, and other products, the U.S. Food and Drug Administration (FDA) faces decisions that may have public-health consequences every day. Often the decisions must be made quic ... more
- Language:
 English
English - Binding: Paperback
- Number of pages: 206
Publisher: National Academies Press, 2011
- More about this

53.37 €
Availability:
50/50 We think title might be available. Upon your order we will do our best to get it within 6 weeks.
We think title might be available. Upon your order we will do our best to get it within 6 weeks.We search the world
You might also like
-

Three-Body Problem
126.67 € -

Shaping of Middle-earth
11.62 € -21 % -

Nobody Ever Told Me (Or My Mother) That!
19.70 € -24 % -

Doing Research That Is Useful for Theory and Practice
67.52 € -

Firumbras and Otuel and Roland
56.50 € -

Building Content Literacy
43.87 € -

Inclusive New Testament
65.40 €
Give this book as a present today
- Order book and choose Gift Order.
- We will send you book gift voucher at once. You can give it out to anyone.
- Book will be send to donee, nothing more to care about.
Availability alert
Enter your e-mail address and once book will be available,
we will send you a message. It's that simple.
More about Risk-Characterization Framework for Decision-Making at the Food and Drug Administration
You get 134 loyalty points
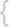 Book synopsis
Book synopsis
With the responsibility to ensure the safety of food, drugs, and other products, the U.S. Food and Drug Administration (FDA) faces decisions that may have public-health consequences every day. Often the decisions must be made quickly and on the basis of incomplete information. FDA recognized that collecting and evaluating information on the risks posed by the regulated products in a systematic manner would aid in its decision-making process. Consequently, FDA and the Department of Health and Human Services (DHHS) asked the National Research Council (NRC) to develop a conceptual model that could evaluate products or product categories that FDA regulates and provide information on the potential health consequences associated with them. A Risk-Characterization Framework for Decision-Making at the Food and Drug Administration describes the proposed risk-characterization framework that can be used to evaluate, compare, and communicate the public-health consequences of decisions concerning a wide variety of products. The framework presented in this report is intended to complement other risk-based approaches that are in use and under development at FDA, not replace them. It provides a common language for describing potential public-health consequences of decisions, is designed to have wide applicability among all FDA centers, and draws extensively on the well-vetted risk literature to define the relevant health dimensions for decision-making at the FDA. The report illustrates the use of that framework with several case studies, and provides conclusions and recommendations.
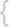 Book details
Book details
Book category Książki po angielsku Medicine Medicine: general issues Public health & preventive medicine
53.37 €
- Full title: Risk-Characterization Framework for Decision-Making at the Food and Drug Administration
- Author: Committee on Ranking FDA Product Categories Based on Health Consequences, Phase II, Board on Environmental Studies and Toxicology, Institute of Medici
- Language:
 English
English - Binding: Paperback
- Number of pages: 206
- EAN: 9780309212809
- ISBN: 0309212804
- ID: 04579244
- Publisher: National Academies Press
- Weight: 416 g
- Dimensions: 229 × 152 × 12 mm
- Date of publishing: 26. June 2011
Trending among others
-

Glucose Revolution
17.28 € -29 % -

The Art & Science of Foodpairing
41.14 € -15 % -

Rewire Your Anxious Brain
17.78 € -11 % -

Clever Guts Diet
11.31 € -28 % -

Gut
8.99 € -27 % -

Why We Eat (Too Much)
11.41 € -20 % -

Encyclopedia of Bach Flower Therapy
28.60 € -19 % -

Miller's Review of Critical Vaccine Studies
18.79 € -18 % -

Nutrition and Physical Degeneration
36.89 € -
Neurosonology and Neuroimaging of Stroke
190.46 € -4 % -

Energy Paradox
23.95 € -15 % -

Worry Trick
16.97 € -19 % -

Gordis Epidemiology
54.18 € -1 % -

Cure Tooth Decay
37.19 € -

Motivational Interviewing in Nutrition and Fitness
38.10 € -

Spillover
12.22 € -23 % -

Yes, You Can Get Pregnant
15.26 € -23 % -

After Cancer Care
14.95 € -22 % -

Fortify Your Life
20.01 € -26 % -

China Study: Revised and Expanded Edition
16.37 € -11 % -

Oxford Handbook of Clinical Medicine
60.95 € -

Nutrition and Physical Degeneration
35.27 € -

It's All in Your Head
11.01 € -23 % -

Close Your Mouth
13.23 € -22 % -

Decision Modelling for Health Economic Evaluation
81.58 € -

Manual of Dietetic Practice 6e & Dietetic Case Studies Set
147.19 € -4 % -

Body Awareness as Healing Therapy
15.26 € -28 % -

Bio-identical Hormones and Telomerase
19.70 € -24 % -

Salt Fix
15.26 € -28 % -

Inner Level
11.31 € -28 % -

I Think You'll Find It's a Bit More Complicated Than That
14.35 € -22 % -

Cancer Revolution
15.05 € -29 % -

Evidence-Based Medicine
42.15 € -4 % -

Causal Inference for Statistics, Social, and Biomedical Sciences
64.79 € -2 % -

Never Too Late to go Vegan
14.24 € -21 % -

Integrative and Functional Medical Nutrition Therapy
149.01 € -4 % -

Nutritional Psychiatry
46.90 € -

Nutrition for Sport and Exercise - A Practical Guide
61.56 € -

Nutrition for Sport, Fitness and Health
132.43 € -10 % -

Nutrition Essentials for Mental Health
46.90 € -6 % -

Clinical Studies in Medical Biochemistry
65.91 € -

Mikronährstoff-Räuber: Metformin
5.04 € -

Marijuana Medicine
26.48 € -4 % -
WHO classification of tumours of haematopoietic and lymphoid tissues
227.66 € -

Bella Figura
11.31 € -28 % -

Proteinaholic
13.64 € -20 % -

Vaccines, Autoimmunity, and the Changing Nature of Childhood Illness
19.30 € -28 % -

Divided Mind
17.99 € -18 % -

Biofeedback
92.39 €
Osobní odběr Bratislava a 2642 dalších
Copyright ©2008-24 najlacnejsie-knihy.sk Wszelkie prawa zastrzeżonePrywatnieCookies


 Vrácení do měsíce
Vrácení do měsíce Zdarma od 49.99 €
Zdarma od 49.99 € 02/210 210 99 (8-15.30h)
02/210 210 99 (8-15.30h)